Physics Assignment Answers
Ch 38 - The Atom and the Quantum
Ch 38 Review Answers:
- In physics, a model describes a way to imagine or visualize (picture) a concept or phenomenon. Often, a scientific theory is called a model because, at least in classical physics, a theory is considered to be an explanation of why something happens the way it does. For example, two models for the nature of light are "Light is made of particles." and "Light is made of waves." Both models give you a way to picture why light behaves the way it does.
In quantum physics, however, you learn that sometimes light behaves like a particle and sometimes it behaves like a wave. Neither simple model of light can be wholeheartedly accepted. In fact, quantum theory does not provide a classical model for light at all! In fact, physicists Neils Bohr, Werner Heisenberg, and others created the "Copenhagen Interpretation of Quantum Mechanics" in 1927-1928 which basically says that no visualization of atomic process is possible! So it has been said that "Quantum physics is the first physical theory that doesn't actually explain anything!" In other words, a classical (pre- 20th century) physicist would probably consider quantum theory more descriptive (law-like), than explanatory (theory-like).
- A quantum is an indivisible unit of something - the smallest "package that it comes in." There are many examples: a photon of light, a molecule of water (in the sense that if you divide it, you don't have water anymore). Cash is quantized - the penny is the smallest unit of U.S. currency. You can't get half a penny in change.
- A quantum of light is called a photon.
- Planck's constant, h, is the ratio of the energy of a photon to its frequency: E = hf, so h = E/f. Planck's constant, therefore, determines how much energy is carried by a photon of light. Since Planck's constant has a very tiny value (It's value, which you really don't need to know, is about 6.67 x 10-34 J.s), which means that quanta of energy are very, very, tiny. We don't notice the "graininess" of energy on human scales, but on atomic scales it becomes very important.
- Since E = hf, higher-frequency photons have more energy than lower-frequency photons. Since the frequency of blue light is higher, photons of blue light have more energy than photons of red light.
- In the photoelectric effect, some metals eject electrons (create an electric current) when exposed to light.
- Photons of blue light have more energy than photons of red light (E = hf), so "blue photons" may have enough energy to knock an electron out of a metal, while a "red photon" does not.
- Yes, bright blue light will eject more electrons than dim blue light because bright light has more photons than dim light, and it only takes one photon to knock an electron out of an atom.
- The photoelectric effect supports the particle model of light.
- a. Yes. The wavelength of a particle depends on its momentum (
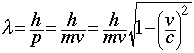 ).
).
b. de Broglie (pronounced close to "da Broy") in 1924
- The de Broglie equation
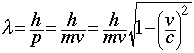 says that wavelength is inversely proportional to velocity, so as the velocity of a particle increases, its wavelength decreases.
says that wavelength is inversely proportional to velocity, so as the velocity of a particle increases, its wavelength decreases.
- The diffraction (interference pattern) of an electron beam supports the wave model of electrons. Particles don't make interference patterns!
- The energy of a photon emitted by an atom is exactly equal to the difference in energy levels of the atom.
- When you say "electrons occupy discrete energy levels in an atom" you mean that electrons can possess only certain amounts of energy within the atom.
- Using the standing wave explanation of electron energy levels in an atom means that the wave model of electrons better explains discrete energy levels in an atom.
- Electrons can occupy energy levels at which the electron waves interfere constructively. Electrons cannot occupy energy levels at which their electron waves interfere destructively. See Fig 38-9 and 38-10 on p. 602 in the text.
- Helium is a smaller atom than hydrogen because the extra positive charge in the helium nucleus pulls the electrons in tighter than the single proton in the hydrogen nucleus pulls its electron.
- Heavier atoms are not tremendously larger than lighter atoms because the extra proton's electric charge pulls the electrons of the heavier atoms closer to the nucleus.
- Quantum mechanics is the physics of the atom and the nucleus, the realm where the quantization of energy becomes a very big factor in the behavior of matter and energy.
- Heisenberg's Uncertainty Principle (1927) says that the momentum (velocity) and position of a particle cannot both be measured with arbitrary precision. If you know the momentum of an electron very precisely, your knowledge of where the electron is located must be very imprecise. If you know the position of an electron with great precision, then your knowledge of the electron's momentum and velocity is very limited.
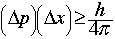 says (uncertainty in momentum)(uncertainty in position)
says (uncertainty in momentum)(uncertainty in position) 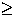 Planck's constant over 4
Planck's constant over 4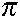 .
.
The Uncertainty Principle applies equally well to energy and time. This has enormous implications in quantum physics which we will discuss later.
Ch 38 Think & Explain Answers:
- To say that a certain quantity is quantized means that it comes in "grains" or "packets" that are the smallest quantities of that substance that it is possible to deal with. Values of a quantized quantity are discrete rather than continuous. See Ch 38 Review #2 above.
- Evidence for the wave nature of light: diffraction (interference) patterns
Evidence for the particle nature of light: the photoelectric effect
- A very bright source of red light consists of very many low-energy photons. The low-energy photon does not have enough energy to knock an electron out of an atom, much like a ping-pong ball thrown at a person does not have enough energy to knock the person off of a chair. Throwing a lot of ping-pong balls at a person is still not going to knock them off the chair (although it might make them pretty mad)!
The model of throwing ping-pong balls at a person is, of course, not exactly the same as shooting photons of red light at an electron, but it has some instructive features. One problem with the model is that a person can be hit by lots of ping-pong balls at once, but an electron only interacts with a single photon at a time. If the photon does not have enough energy to eject the electron completely from the atom (like a red-light photon) the electron will either simply (a) ignore the photon if its energy doesn't match a permitted energy jump for the electron, or (b) absorb the photon and assume the higher energy level, then immediately emit a photon to return to a lower-energy state.
- Since the energy of a photon is given by E = hf, the highest-frequency light has the most energetic photons. Therefore, ultraviolet photons are the most energetic of the three, since ultraviolet light has the highest frequency.
- Green light has a higher frequency than red light, so green photons are more energetic than red photons (E = hf). Therefore, it would take more red photons than green photons to give the same energy. (Imagine red photons are pennies and green photons are nickels. How many of each would it take to make a dollar?)
- Ultraviolet light has a higher frequency than infrared light. Therefore sun tanning produces cell damage because ultraviolet photons are (much) more energetic than infrared photons (E = hf). This damage is produced by ultraviolet photons knocking not just electrons (which produces oxidation - burning) but nucleons out of your atoms (which changes their chemical nature).
- The de Broglie equation (
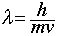 ) says that a particle's wavelength is inversely proportional to the particle's velocity. Therefore, the slower-moving electrons will have the longer wavelength.
) says that a particle's wavelength is inversely proportional to the particle's velocity. Therefore, the slower-moving electrons will have the longer wavelength.
- We don't notice the wavelength of moving matter in our day-to-day lives because the wavelengths are so small. For example, suppose a 60 kg person is moving down the hallway at 1 m/s. Their de Broglie wavelength would be:
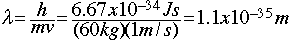
This is thirty-five orders of magnitude smaller than a typical atom!
- In E = hf, the energy of a photon is proportional to Planck's constant. Therefore, if Planck's constant were larger the energy of a photon would be larger. It is the extremely-tiny value of Planck's constant that keeps us from noticing the quantum nature of energy on human scales.
- Helium gas will leak from a balloon faster than hydrogen gas for two reasons. First of all, the two protons in the helium nucleus pull the helium atom's electrons closer to the nucleus than hydrogen's one nuclear proton does - the two protons exert twice the electric force than a single proton. This means that the helium atom is smaller than the hydrogen atom.
A second reason relates to the chemistry of helium and hydrogen. Hydrogen forms diatomic (two-atom) molecules, while helium is chemically inert - helium atoms do not readily combine chemically with any other atoms. Therefore, hydrogen gas not only has bigger atoms, but they are joined together in pairs, while helium gas consists of single, smaller atoms.
last update May 18, 2009 by JL Stanbrough
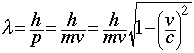 ).
).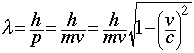 says that wavelength is inversely proportional to velocity, so as the velocity of a particle increases, its wavelength decreases.
says that wavelength is inversely proportional to velocity, so as the velocity of a particle increases, its wavelength decreases.